No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
Medical Device Regulation
The new European Medical Device Regulation (MDR) with many new rules is a challenge for all involved. B. Braun is preparing intensely and wants to apply the new requirements as soon as possible. Here we have compiled some information about the MDR for you.
The new European Medical Device Regulation (MDR) went into force in May 2017. The new regulation replaces the existing Medical Device Directive (MDD) and the Active Implantable Medical Device Directive (AIMDD).
The current approx. 500,000 medical devices in Europe will be recertified in accordance with the new, substantially more comprehensive policy to receive the CE mark. According to current estimates, approximately only 65 percent of the medical devices will be certified according to the new regulation. Some notified bodies are still in the designation phase and it is not fully clear as to how many notified bodies will be able to conclude the process (list on European Commission website). Due to the increased requirements placed on the notified bodies and the manufacturers, portfolio adjustments are expected and are inevitable.
MDD certificate “grace period”: No design change for MDD certified products
/
Products must have a MDR application signed (except for MDD products being sold out)
/
MDD certificates maintain validity (timeline depends on product risk class)
/
Among others, these areas are affected:
In order to be able to ensure continuous supply with safe and innovative medical technology, all manufacturers are faced with the challenging task of overcoming the increased requirements necessary to receive the CE mark. The notified bodies must lay the foundation and create sufficient capacity for the conformity assessment procedure.
At an earlier point in time, B. Braun already began wide-ranging preparations for the certification of its own medical devices in accordance with the MDR. Naturally, this applies to all products that B. Braun manufactures itself or purchases as commercial goods to complete its portfolio. With regard to the progress of the measures taken, B. Braun is confident it will be able to comply with the requirements of the MDR until May 2024.
A revision of the Medical Device Directive 93/42 EEC (MDD), which was made public in 1993 and still applies today, became necessary at the European level. With the new regulation, EU authorities would like to improve the quality of medical devices and enhance safety, harmonize the processes through the EU, and increase patient safety. Additional aspects include the improvement of transparency and traceability in connection with new technologies that allow for the clear identification of all products throughout their entire lifecycle.
The MDR defines the requirements that a manufacturer must meet in order to sell medical devices in Europe. Both the technical requirements for a product and the requirements placed on the monitoring of products used in health care facilities are affected.
There are several changes surrounding the classification of products. In addition to the introduction of the new class lr for reusable surgical instruments, the requirements placed on implantable products of class IIb have been especially increased. Furthermore, numerous product categories have been assigned to a higher risk class. The MDR increases the requirements pertaining to the clinical evidence of medical devices. In the future, all medical devices, regardless of their risk class, will require a clinical evaluation. The newly introduced scrutiny procedure means the improved monitoring of new, implantable products of risk class III, as well as active medical devices which administer and/or remove medicinals of Class IIb before market launch. In addition to the increased requirements placed on manufacturers, there are now stricter rules imposed on notified bodies. In order to be able to approve medical devices, various additional requirements must be met. Also, the notified bodies are obliged to perform unannounced audits at manufacturers. Additional requirements placed on the technical documentation that must be provided by manufacturers is substantially increasing the extent and complexity of the documentation.
There is no exact identifier on the product to indicate MDR compliance. Nevertheless, a new ISO symbol “MD” for medical devices will be placed on the label along with the introduction of the MDR.
Yes, all medical devices of all risk classes, including the treatment units and systems, are affected.
Data that is relevant for the public is made accessible in a central European database that already exists today. The expanded version of EUDAMED will be introduced stepwise, i.e. voluntary usage of a “minimal viable product” (MVP) version in 2022. Mandatory usage of the EUDAMED is expected to be in Q2 2025. See EUDAMED time line
Legal manufacturers, system and procedure pack producers, importers, or authorized representatives for the distribution of medical devices in the EU must add to EUDAMED data pertaining to the role of the actor, as well as the product-relevant data for each individual product to be distributed in the EU.
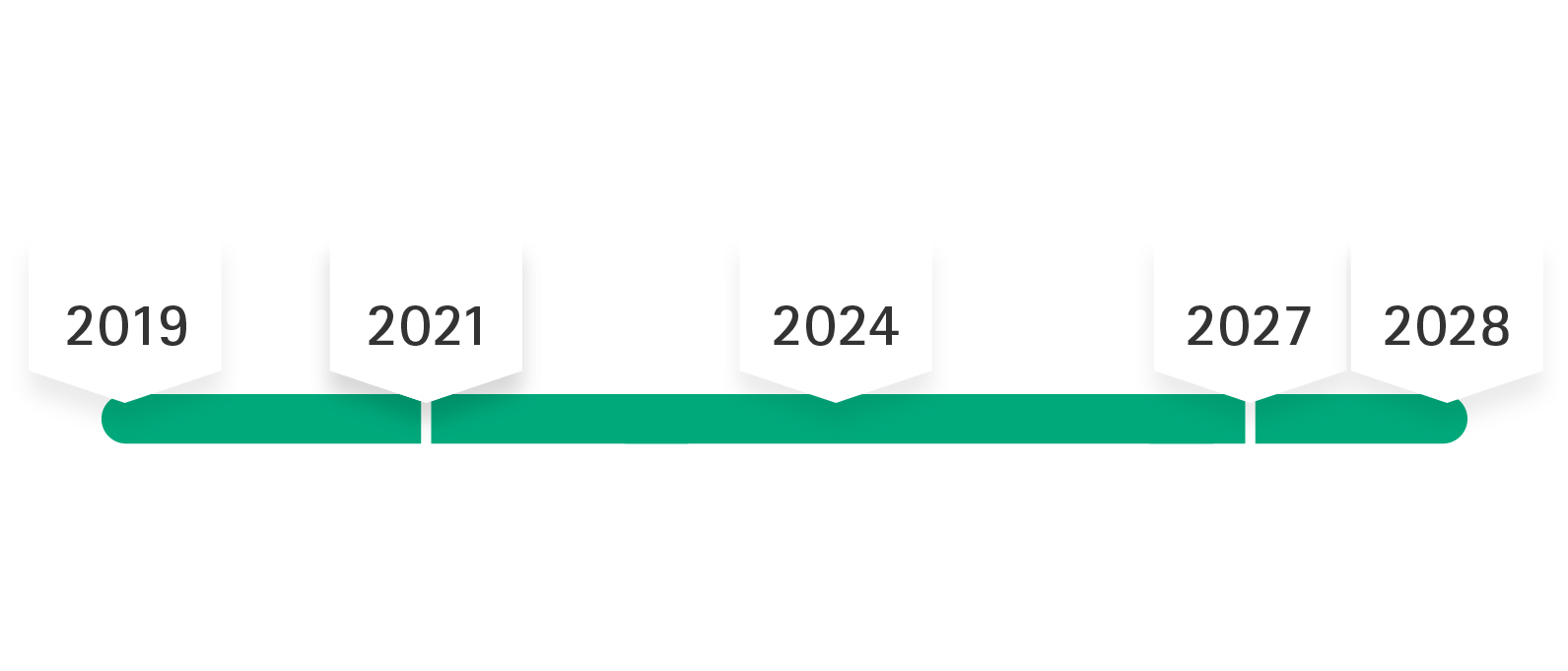
After the MDR was published on May 5th, 2017, the regulation went into force on May 25th, 2017, with a transition period that was intended to last until May 26th, 2020. Due to the COVID-19 pandemic the mandatory application of the MDR was postponed to May 2021. According to the 2nd amendment to MDR the transition time is extended until max. December 2028, i.e. MDD certificates will maintain their validity (i.e. only until max. December 2027 for products of risk classes IIb implantable and III), if certain prerequisites are met.
Medical devices shall not present any unacceptable risk to health and safety.
QMS shall be already MDR compliant.
MDR application is signed by May 2024.
MDR contract with the Notified Body shall be signed by September 2024.
no significant changes to be applied to MDD products.
According to the 2nd amendment to MDR no final sell-off period exists any more. The previously planned sell-off period (i.e. no further distribution of MDD certified products after May 2025) has been cancelled.
MDD EC certificate validity extended automatically (no new certificates to be issued).
MDD and MDR EC certificates will be valid in parallel over the period of the timeline extension.
Both, MDD and MDR products can be placed on the market in parallel.
Due to the COVID-19 pandemic, the mandatory application for MDR was set for May 26th, 2021. This results in the following deadlines for the marketing of medical devices according to product classes:
Class I: May 26th, 2021
Class Ir, s, m, Class IIa, Class IIb and Class III: May 26th, 2024
The conformity assessment says whether a product and the respective manufacturer complies with the European MDR requirements. Depending on the risk classification of the individual products, B. Braun is entitled to carry out this audit itself. The additional evaluations are carried out via a so-called “notified body”.
A notified body is a private company that is named on behalf of the European Union to evaluate the conformity of a manufacturer with the MDR. Currently they are being evaluated with audits and after they have passed the audits, the notified bodies will then evaluate the processes of medical device manufacturers for MDR conformity. As soon as the compliance of the processes has been verified, products that are processed can be registered through these processes.
Even after converting the entire product portfolio to MDR, manufacturers will face significant additional costs due to the increased requirements of the MDR.
The main objectives of the regulation are better protection of public health and patient safety, more transparency, legal security and a more European-oriented concept. This is to be achieved through more extensive technical documentation on the affected products in an MDR-compliant quality management system.
Regulation (EU) 2023/607 provides that certificates issued under the MDD by May 25, 2017 and still valid on May 26, 2021 remain valid regardless of their expiration date. This means that products for which an MDD declaration of conformity was valid until May 26, 2021 may continue to be placed on the EU market.
Products that were already on the market before May 26, 2021 and had an MDD declaration of conformity can continue to be placed on the market as long as the MDD declaration of conformity is valid. The extended validity applies until May 2027 (for risk classes III and IIb implantable) and until May 2028 (for all other risk classes - except class I).
Regulation (EU) 2023/607 abolished the sell-off period for MDD products, which was originally set to expire on May 27, 2025.
The manufacturer confirms with the manufacturer's own declaration (confirmation letter) that its medical device complies with the applicable legal requirements. According to Regulation (EU) 2023/607, the validity of all certificates that had not expired on March 20, 2023 was extended until December 31, 2027 (for products in classes III and IIb implantable) or until December 31, 2028 (for all other products - except class I). The manufacturer must fulfill the following conditions for the continued validity of the certificates and declarations of conformity.
As a manufacturer of medical devices, B. Braun must comply with the requirements of the MDR. Different work groups are updating the technical documentation and revising processes in order to ensure they are MDR compliant. Furthermore, B. Braun is obliged to provide product information, including unique device identification data (UDI), as well as post market surveillance information to EUDAMED.
The B. Braun Group has initiated comprehensive measures and provided resources to ensure the on-schedule implementation of the MDR.
All medical devices.
For some time, B. Braun has been preparing itself intensively for the new regulations and assumes it will be able to adhere to the timeline.
The quality management systems of B. Braun Melsungen AG, B. Braun Avitum AG and Aesculap AG have already been certified in accordance with the MDR and MDR-compliant technical documentations for our products have already been prepared . The product transfer to MDR will take place successively up to the maximum term.
Generally speaking, yes – depending on the planned lifecycle, the products are certified according to MDR. As in previous years, B. Braun will continue to make adjustments to its product portfolio. New products will be added to the portfolio and older generations will be replaced, or uneconomical or outdated products will be removed from the product portfolio. As part of our regular assortment management process, we will continue to communicate corresponding product assortment changes in a timely, open, transparent and targeted manner to the respective customers affected and offer alternatives where possible.
B. Braun is using the MDR transition period in which we will transfer our product portfolio to MDR. During this transition period, B. Braun will place both MDD and MDR certified products on the market.
TÜV Süd was recognized as the second notified body worldwide in May 2019. Further notified bodies supervising medical devices of B. Braun are MedCert, Dekra and TÜV Rheinland which received their designation under MDR as well. Please check the link to the European Commission’s website to obtain an overview on MDR accredited notified bodies.
European Commission’s website
a) The identification of a medical device will change through the inclusion of the unique device identification (UDI).
b) It is possible that extended documentation obligations arise through new Class III products.
c) The EUDAMED database provides customers more transparency with regard to products.
B. Braun is MDR-ready and has already made high investments in the multi-digit million range to achieve this. Major cost factors are more comprehensive requirements for the technical documentation, technical and systemic solutions to meet the required EUDAMED data management as well as increased post market surveillance efforts.
B. Braun is actively supporting customers in obtaining information. To this end, Declarations of Conformity and Informations for Use (IFU) are made successively available digitally since May 2021.
Declarations of conformity can be found online at www.bbraun.com - simply by entering the product name in the search field and finding the corresponding Declaration of Conformity under the heading -related documents-. Most of the declarations of conformity of B. Braun Melsungen AG and B. Braun Avitum AG can be accessed via this channel. For technical reasons, B. Braun will only be able to make the declarations of conformity for Aesculap AG products (with the exception of the suture material portfolio) available online in the medium term. For the random provision of declarations of conformity, which cannot be found online, the known contacts from the customer service of the B. Braun national subsidiary can be approached.
Instructions for use can be downloaded at https://eifu.bbraun.com After entering the article number or the GTIN, the documents belonging to the product are displayed.
Most of the letters of confirmation are available online at bbraun.com under the respective product documents. If they cannot be found online, they can be requested at the respective sales organization. An overview of country organizations can be found here.
The confirmation letter from the Notified Body refers to Regulation (EU) 2023/607 and is a letter of confirmation. In this letter, the Notified Body confirms that the manufacturer (including the devices concerned) complies with the requirements of this Regulation. These letters of confirmation can be made available via customer service.
The MDR only stipulates that an unambiguous indication of the time limit needs to be provided for using or implanting the device safely, expressed at least in terms of year and month, where this is relevant.
Products from B. Braun on which the best-before date is indicated with the month and year can be used until the end of the month.
Here is a collection of useful links to information on official websites of the European Comission and other sources.
Factsheet for health care professionals (EU website)
link
EU-Journal: Medical Device Regulation
link
List of Notified bodies (EU)
link
EUDAMED timeline (EU)
link
B. Braun Electronic Instructions for Use (eIFU)
link
B. Braun Fact Sheet on MDR
pdf, 53.7 KB
Q&A on practical aspects to MDR transitional provisions
link
Your feedback matters! Participate in our customer survey to help us enhance our website, products and services. Thank you for your support!