No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
Intermittent urinary catheterization
Urinary tract infection (UTI) is one of the most important complication of patients requiring intermittent urinary catheterization[1, 2] and can impact patients' overall health and quality of life.[1, 3]

The emotional and practical burden of intermittent catheterization and UTIs is considerable in the patients’ lives.[3] UTIs account for about 23 to 63 percent of the total lifetime costs for spinal cord injury patients practicing intermittent catheterization.[4] Very important are good education of all involved in IC, good patient compliance, the use of proper material and the application of a good catheterization technique.[2]
UTIs related to intermittent catheterization can have negative consequences such as:
Recurrent urinary tract infections often lead to repeated use of antibiotics and thus can accelerate the development of antibiotic resistance and persistence.[5]
It is therefore very important to reduce the risks of UTIs in patients as much as possible.
Real-World Evidence
We funded the first real-world evidence of intermittent catheterization to compare the effects of pre-lubricated and hydrophilic catheters on the incidence of symptoms suggestive of urinary tract infections (ssUTIs).[6]
A patient database compiled by a representative panel of 1,950 general practitioners and 2,950,381 active patients in the UK was used for this longitudinal, observational study. This database considered 5,296 people using catheters.
Most importantly, the study showed significant results indicating a lower incidence of ssUTIs when pre-lubricated catheters were used:[6]
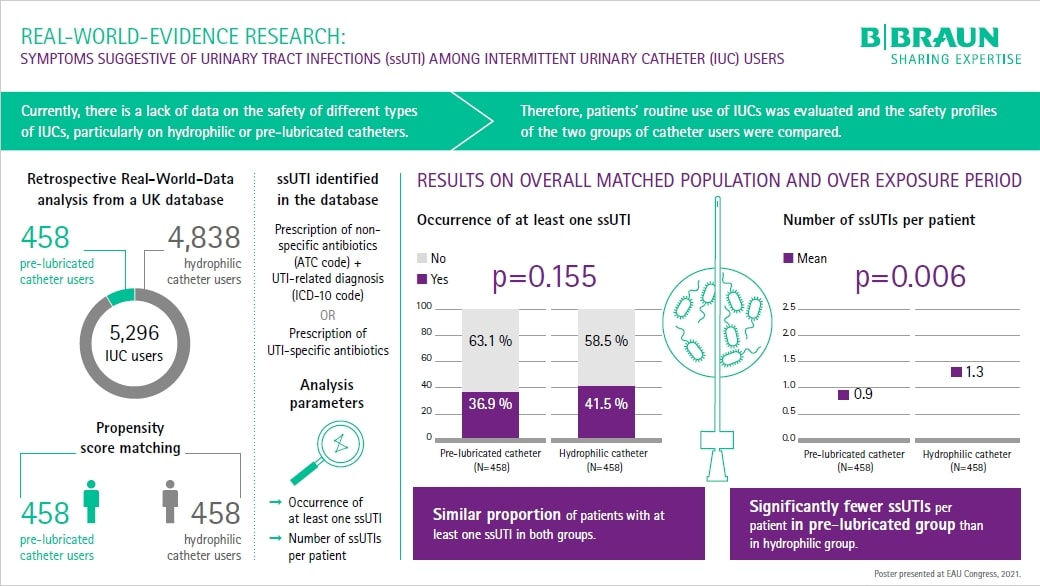 Study design and extract of its outcome, modified by B. Braun for the EAU congress 2021.
Study design and extract of its outcome, modified by B. Braun for the EAU congress 2021.
Professor Chapple (Royal Hallamshire Hospital, Department of Urology, Sheffield, United Kingdom), Professor Chartier Kastler (Sorbonne University, Department of Urology, Paris, France) and Professor Schurch (Vaudois University Hospital of Lausanne, Department of Clinical Neurosciences, Lausanne, Switzerland) participated in and supported this study which was accepted by three different congresses:
Broadly, all data collected routinely (that is, not as a part of randomized controlled trials) on patient health from different sources, are called RWD7.
The US FDA defines Real-World Data as data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources. Examples of RWD include7:
Real-World Evidence is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of Real-World Data (RWD)[7].
Your feedback matters! Participate in our customer survey to help us enhance our website, products and services. Thank you for your support!