No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
No content results match your keyword.
Content
No product results match your keyword.
Products
BASKET-SMALL 2
Basel Stent Kosten Effektivitäts Trial Drug Coated Balloons vs. Drug Eluting Stents in Small Vessel Interventions
Jeger R et al. The Lancet, August 2018.
Trial Registration Number: NCT01574534 on https://www.clinicaltrials.gov
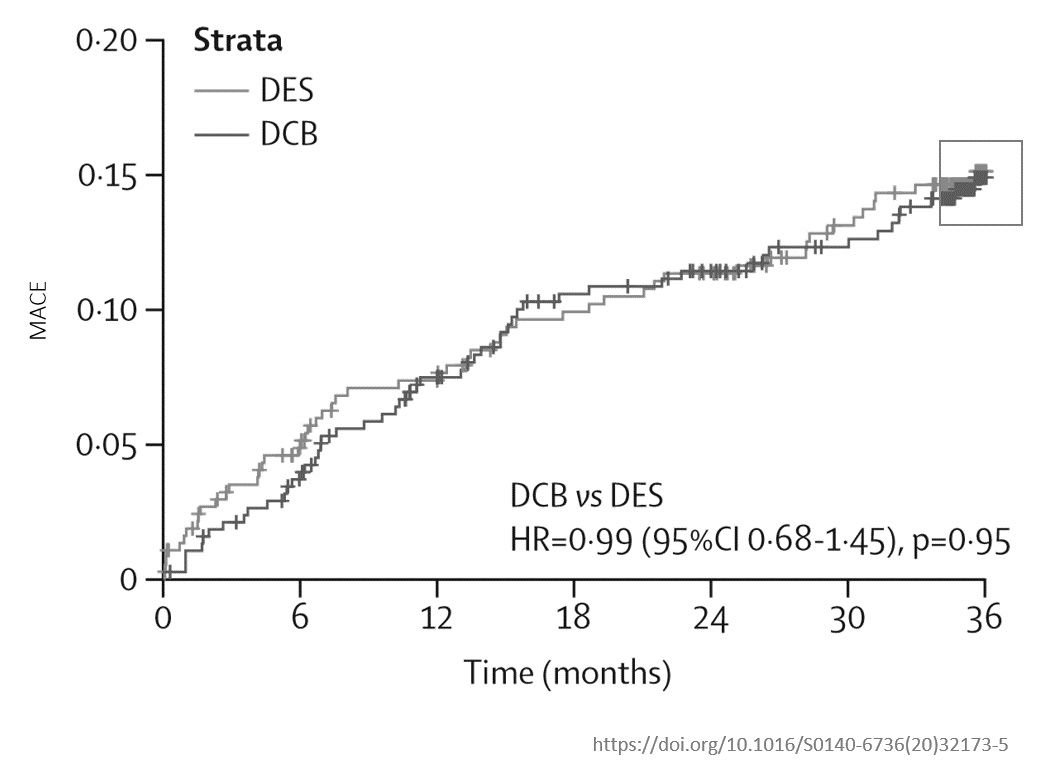
Jeger R et al. The Lancet, October 2020. Same trial registration.
Main inclusion criteria:
Primary outcome:
Secondary outcomes:
Dual Antiplatelet Therapy (DAPT):
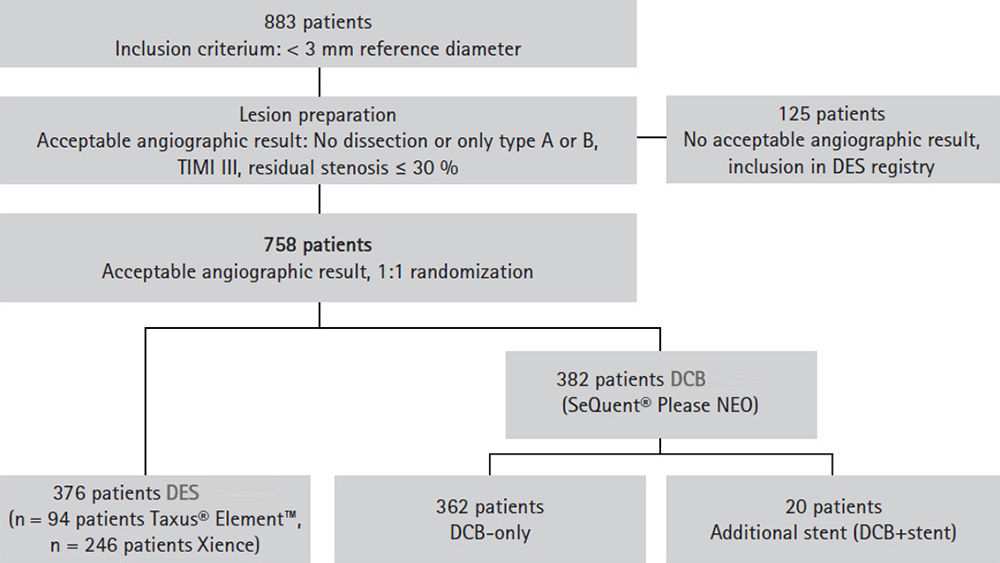
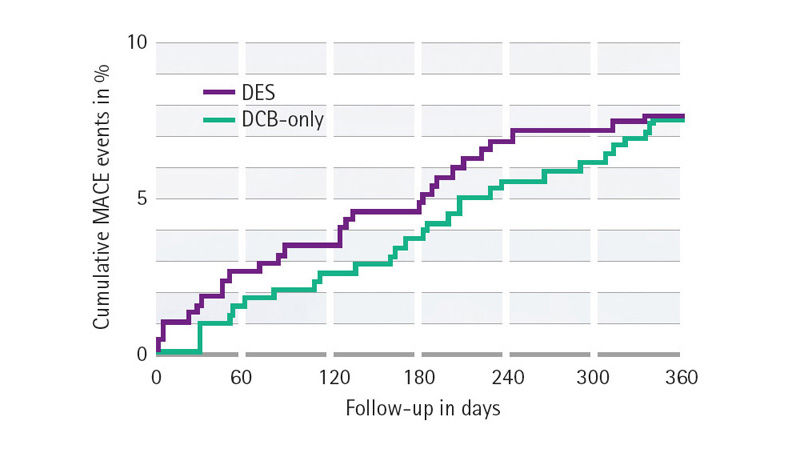
| DCB n = 362 | DES n = 376 | p-value | |
| TVR | 3.4 % | 4.5 % | 0.44 |
| MI | 1.6 % | 3.5 % | 0.11 |
| Cardiac death | 3.1 % | 1.3 % | 0.11 |
| MACE | 7.3 % | 7.5 % | 0.92 |
Your feedback matters! Participate in our customer survey to help us enhance our website, products and services. Thank you for your support!