No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
Advanced Surface Technology
Advanced Surface Technology is an innovative multilayer coating for knee implants that is unique in the market. With improved mechanical properties and fantastic results in biocompatibility – it is an effective solution to prevent complications in primary and revision surgery.
7 layers for protection
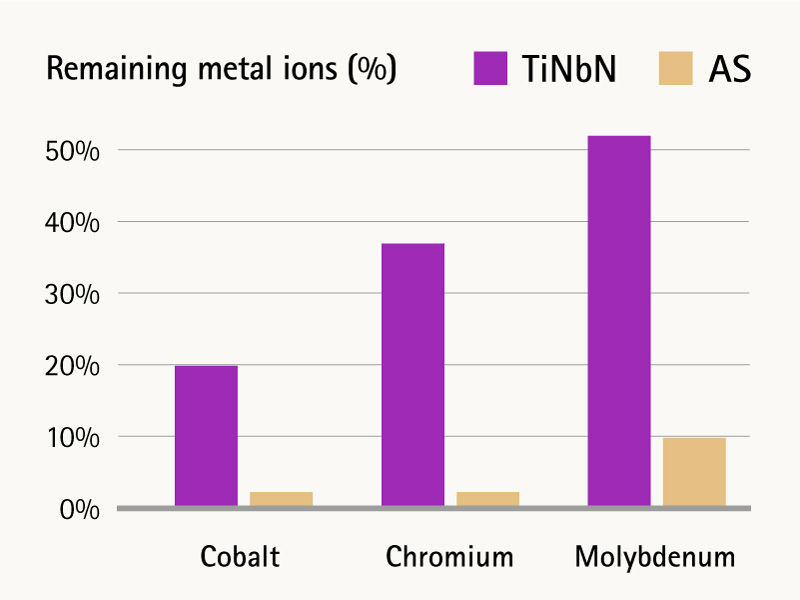
Unlike monolayer coatings, the AS 7-layer coating with Zirconium Nitride surface (ZrN) has a buffer zone which compensates the changes in surface hardness and results in more resilient elastic properties. In addition, the transition layers act as an improved metal ion barrier and offer a more efficient metal ion reduction than monolayer coatings with Titanium Niobium Nitride surface (TiNbN).1, 2
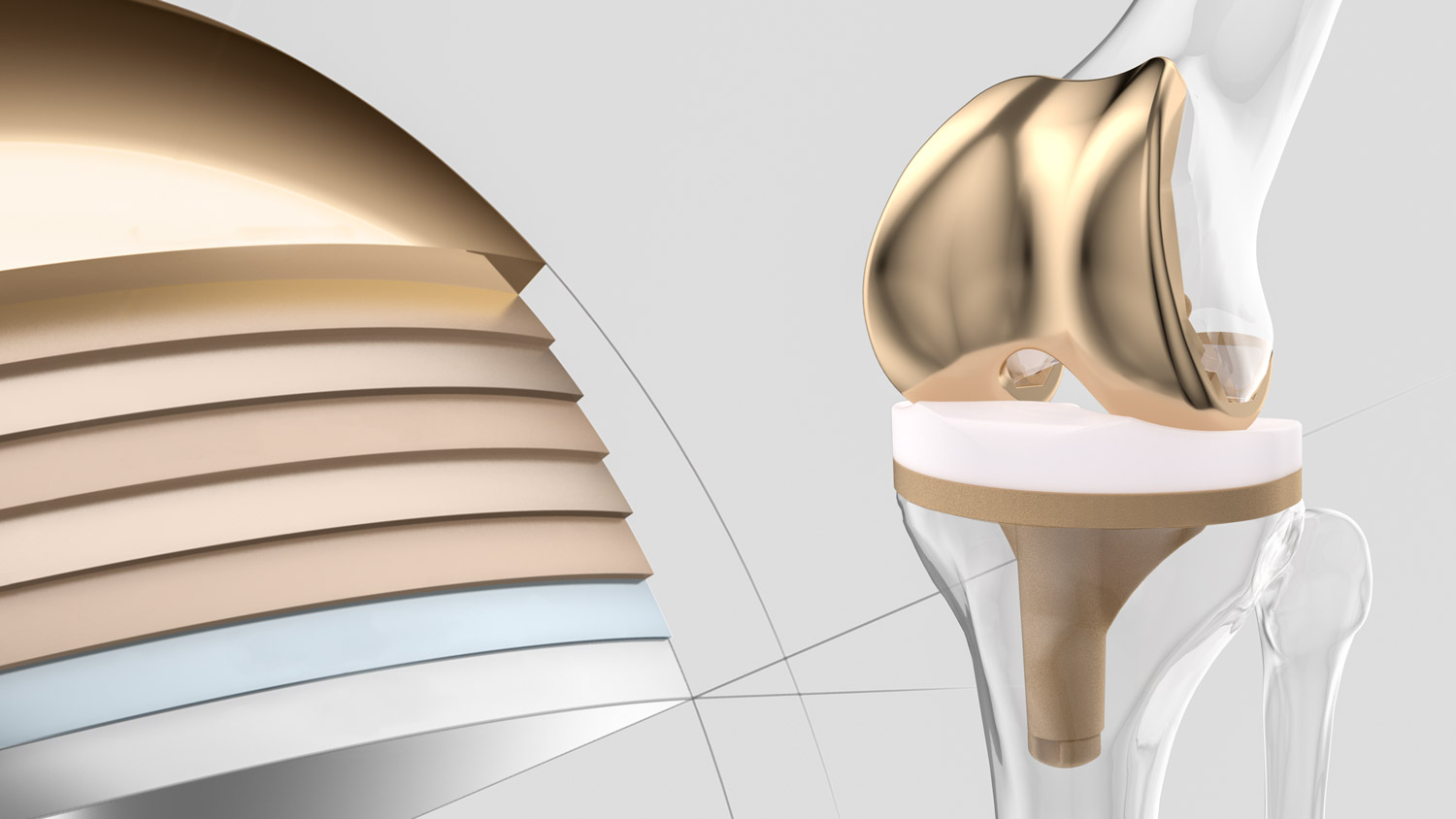
The top layer is made from zirconium nitride, a hard ceramic material. It offers exceptionally high wear resistance. A hard surface increases scratch resistance 2 and ensures lower polyethylene abrasion.
/
The five transition layers provide stability and create a barrier effect. These layers prevent the release of metal ions and bridge the difference in hardness.
/
This adhesive layer provides a firm connection between the implant and the subsequent coated layers.
/
Improving the molecular structure of the layered system. The Advanced Surface multilayer structure provides the coating with high elasticity and prevents an eggshell effect – even under extreme conditions.
/

“I do everything for satisfied patients with good long-term results – so it is a logical consequence for me to use the Advanced Surface Technology implants. The multilayer technology compensates for the changes in the hardness of the surface and provides a safe barrier against the release of metal ions”
Enhanced biocompatibility
It has been discovered that a possible contact allergy to implant materials or the components contained in bone cement may lead to implant intolerance. Endoprostheses are known to release metal ions into the human body. This can lead to immune reactions in patients who are at risk of hypersensitivity. The metal hypersensitivity prevalence among the general population is relatively high at 13%.3 60% of patients with complications after metal implants are sensitive to metals.4
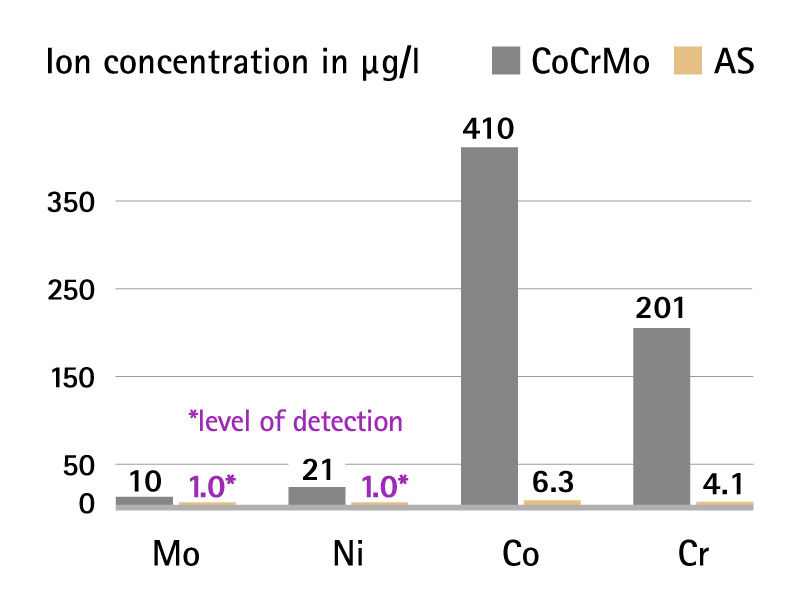
With AS Technology, metal ion concentration is near the level of detection and below biological threshold.2 The material with excellent biocompatibility seals the metallic components, providing an effective metal ion barrier.
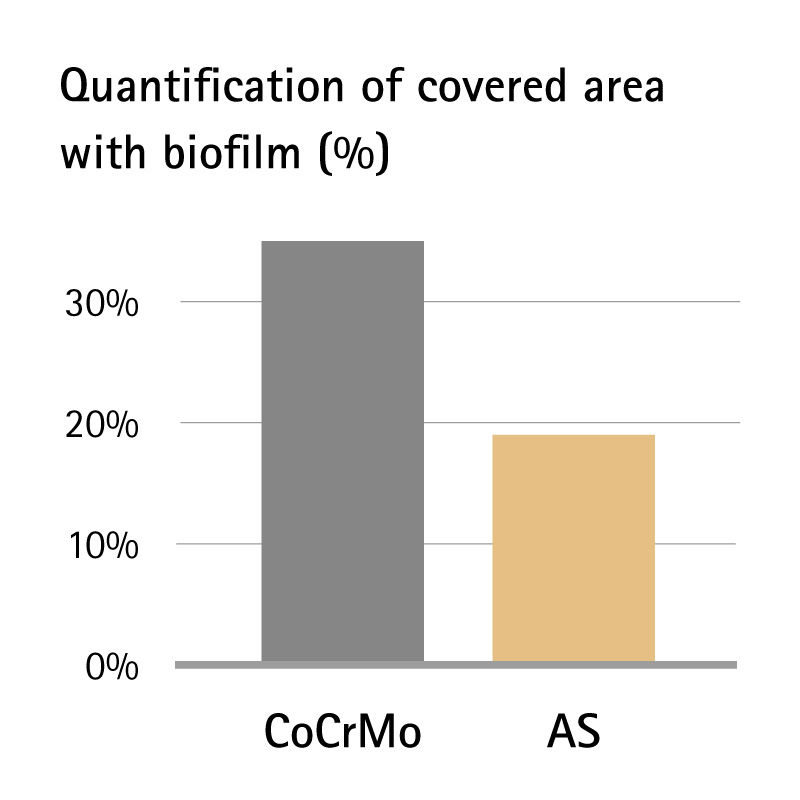
One of the most commonly identified pathogens responsible for orthopaedic implant infection is Staphylococcus epidermidis, which can form biofilms on surfaces and lead to medical problems. The AS surface with zirconium nitride top coat showed 45% lower biofilm formation compared with standard uncoated CoCrMo implants.5
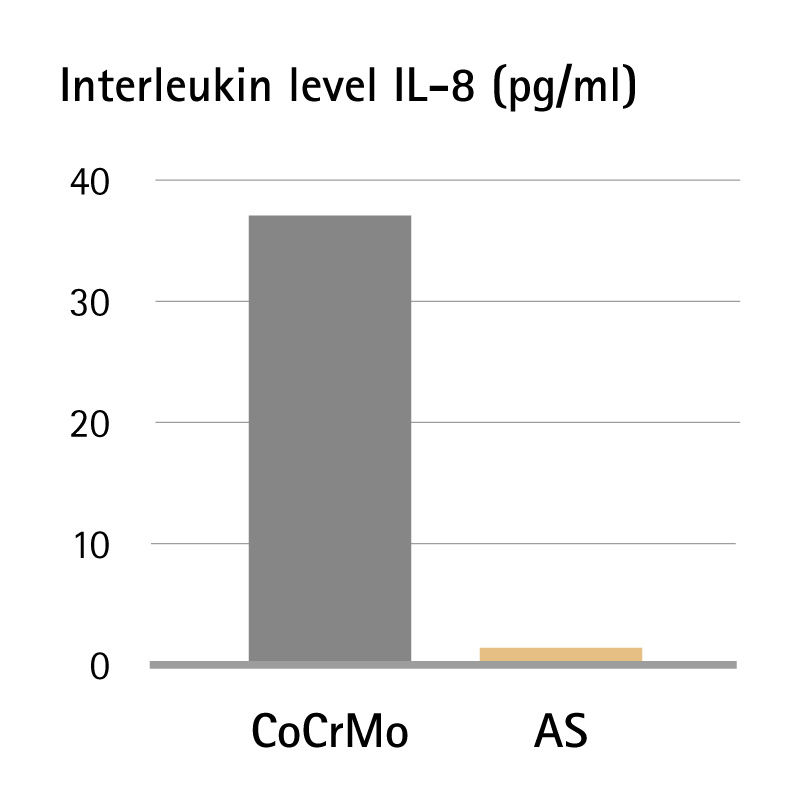
Interleukins (IL-x) are endogenous messenger substances of the cells of the immune system. Elevated levels of interleukin in the blood indicate the presence of an inflammatory reaction. Using implants with Advanced Surface Technology means no additional pro-inflammatory triggering.6
AS wear solution
The wear of the gliding surface is directly linked to aseptic loosening due to osteolysis triggered by polyethylene wear particles. Aseptic loosening is the most common reason for revisions in TKA.7, 8 The interaction of components is therefore very important for the results of a knee surgery and the patient's well being.
The zirconium nitride top coat yields superior surface hardness that is unmatched in the market place. The wear rate is reduced by up to 88% compared to standard implants. This ceramic surface layer stands for improved scratch resistance, good wettability and for better articulation between bearing surfaces.
0%
AS knee implants demonstrate up to 88% reduction in wear when compared to CoCrMo prosthesis.9
The combination of AS Technology for the femur and tibia component and the beta sterilization procedure for our gliding surface yields to superior performance. Beta radiation uses higher concentrated, lower penetrating radiation levels for reduced oxidation im aged material. It combines the advantage of low wear with good mechanical properties of conventional polyethylenes.
0%
Beta PE provides 70% reduction in oxidation levels.10
AS clinical evidence
The clinical performance is very important – not only for the surgeon that aims for a good result after surgery, but also for the patient that needs the confidence to make the right choice for his/her health. There are many different studies and papers to prove the effectiveness and advantages of Advanced Surface Technology.
AS implant portfolio
The AS coating technology can be applied to all implant components. It is important for us to let you decide for your preferred AESCULAP® knee implant system – with the option in AS.

“As it happens, I was a candidate for a knee replacement, and I chose Advanced Surface Technology, which I use on every single patient. Why should I use a lesser item for my patients?”
It will be forwarded to a responsible contact person who will get in touch with you as soon as possible.
Your feedback matters! Participate in our customer survey to help us enhance our website, products and services. Thank you for your support!