No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
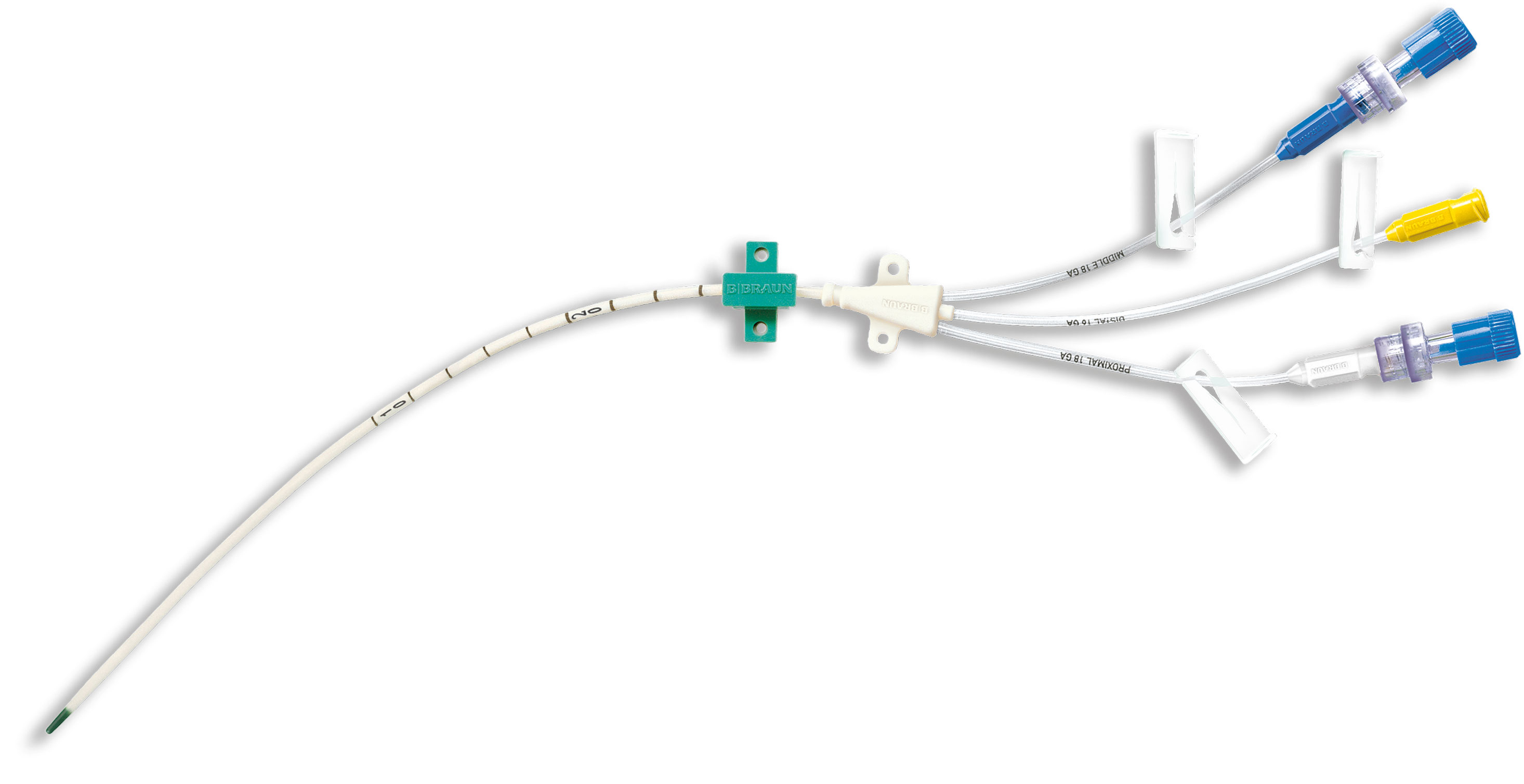
Central venous catheter (CVC) Certofix®
B. Braun offers a comprehensive portfolio of acute central venous catheter sets for both, adult and pediatric patients.
As your system partner for ultrasound-guided central line placement and ECG-guided catheter tip positioning, our goal is to make CVC placement safer
/
To adjust optimal skin fixation position with sutured or non-sutured fixation
/
/
Additionally to the lumen configurations and the non-coated catheters, B. Braun offers a central venous catheter with proven non-leaching antimicrobial effect durable over 30 Days – Certofix® protect.
*Krikava I, et al. The efficacy of a non-leaching antibacterial central venous catheter – a prospective, randomized, double-blind study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020 Jun;164(2):154-160.
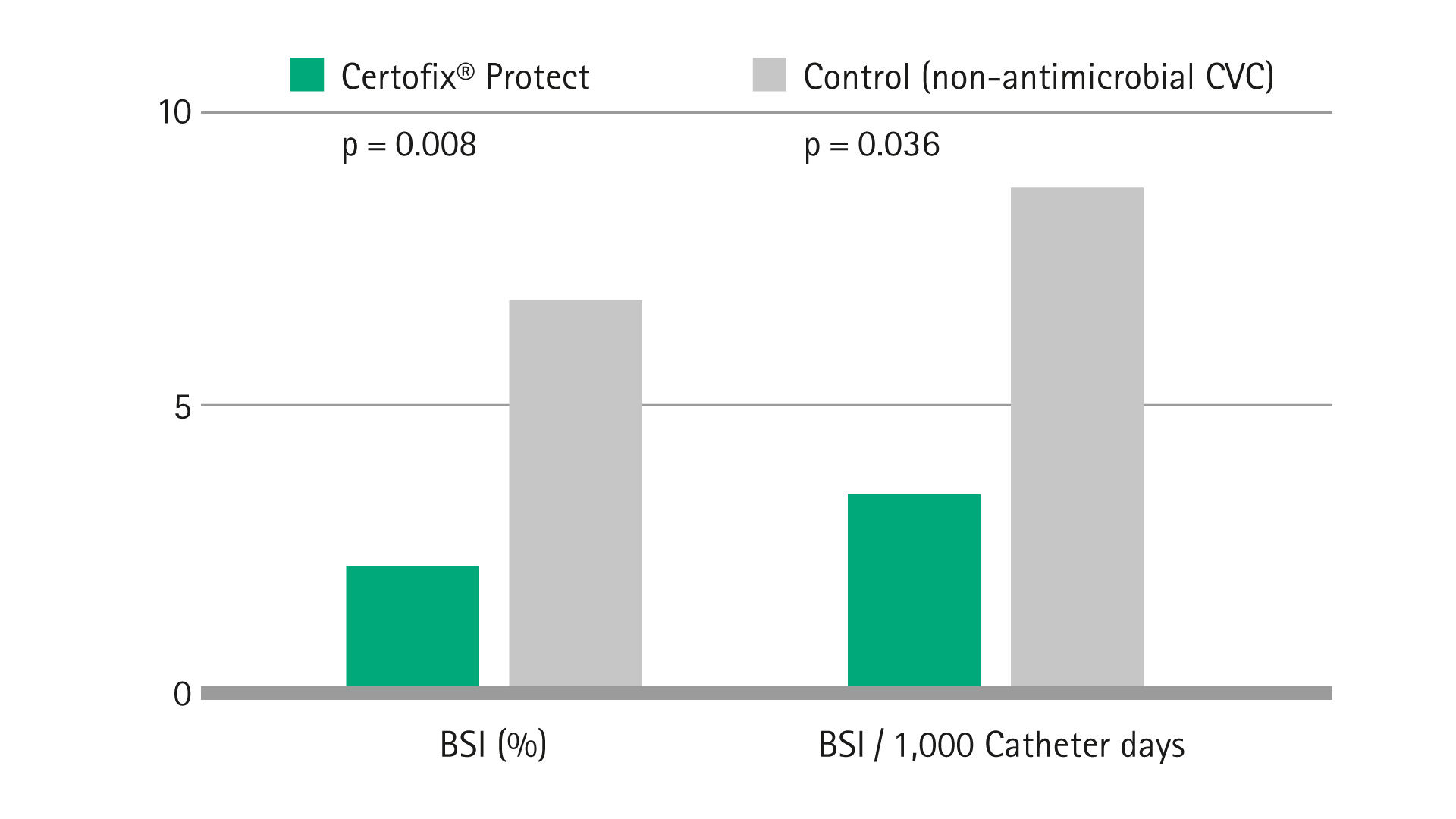
from 4,200€ to
0€
CRBSIs (catheter related blood stream infections) create additional costs per episode ranged
include a number of different components dependent on the individual set configuration, such as introducer needles, a Nitinol guidewire, syringes, scalpels, dilator, ECG cable, different kind of needle-free connectors and fixation devices. B. Braun‘s Certofix® range is complemented by a number of accessories to confirm CVC tip position during or after placement via Intraatrial ECG. Compatible with most commonly used ECG monitors.
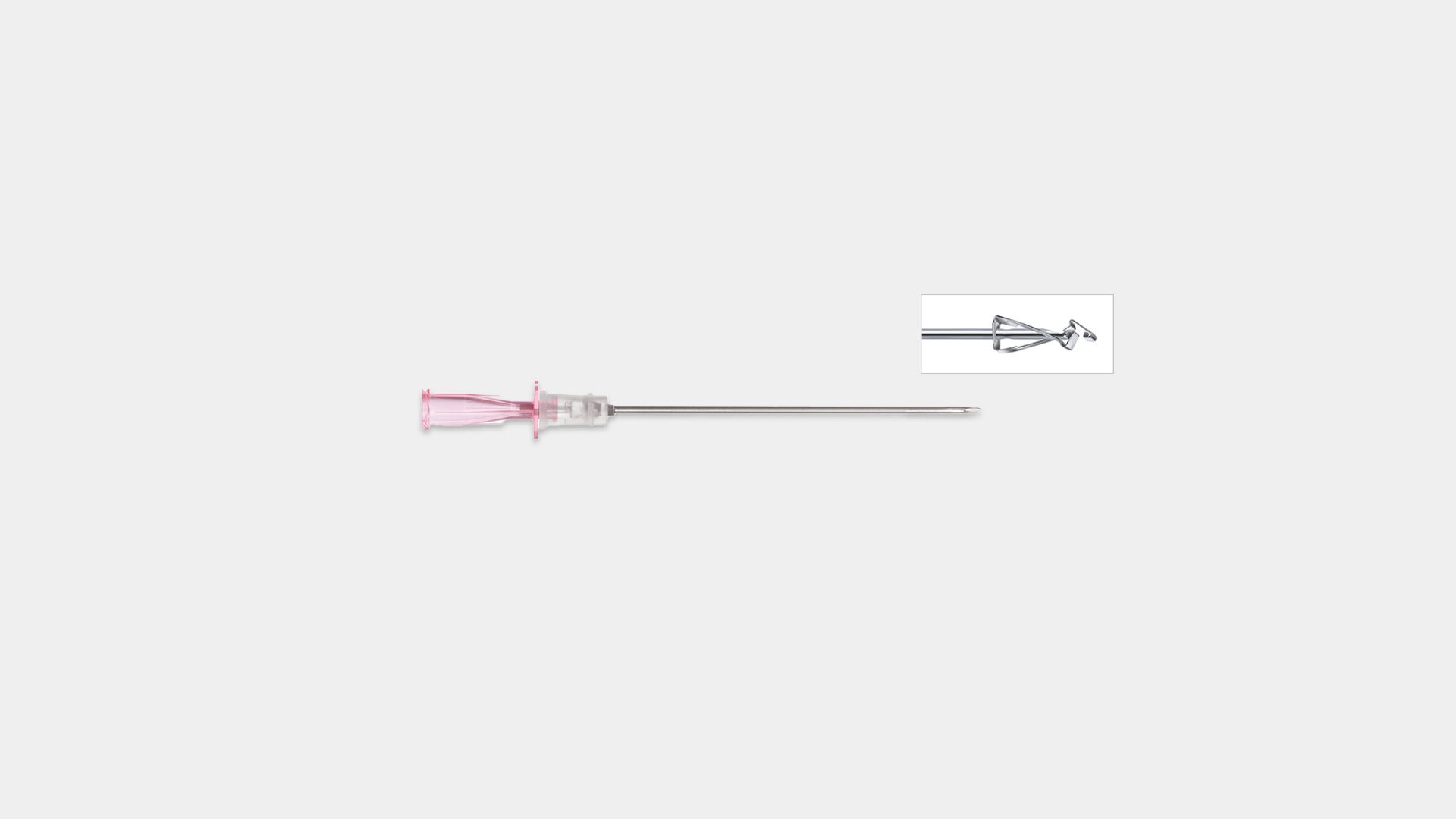
Active safety clip (after use) to prevent needle stick injury
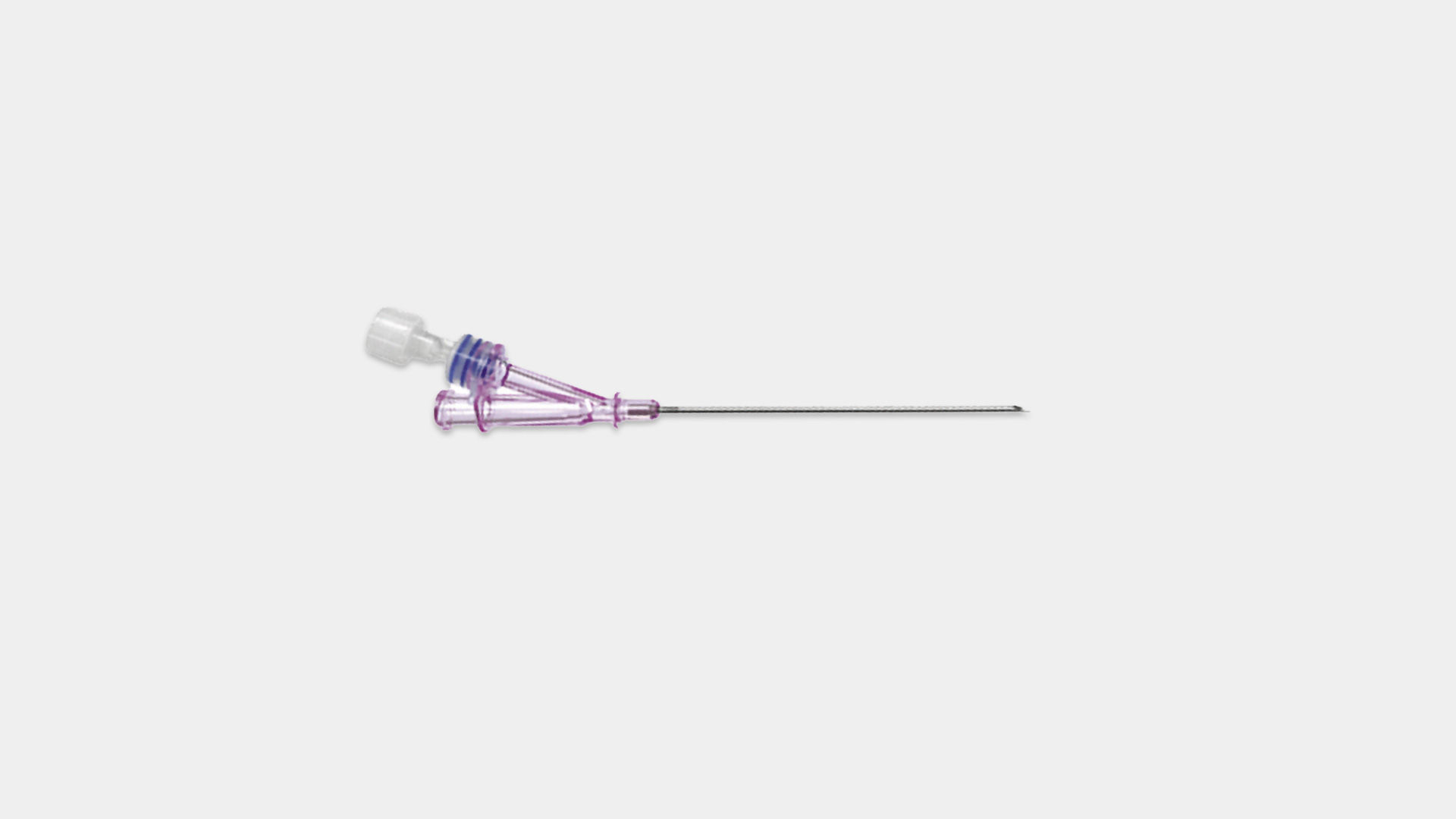
Allows guidewire insertion and aspiration without disconnecting the syringe. Prevents air embolism and excessive blood loss during guidewire insertion.

The scalpel can be slid back into the housing and blocked to prevent sharps injury.
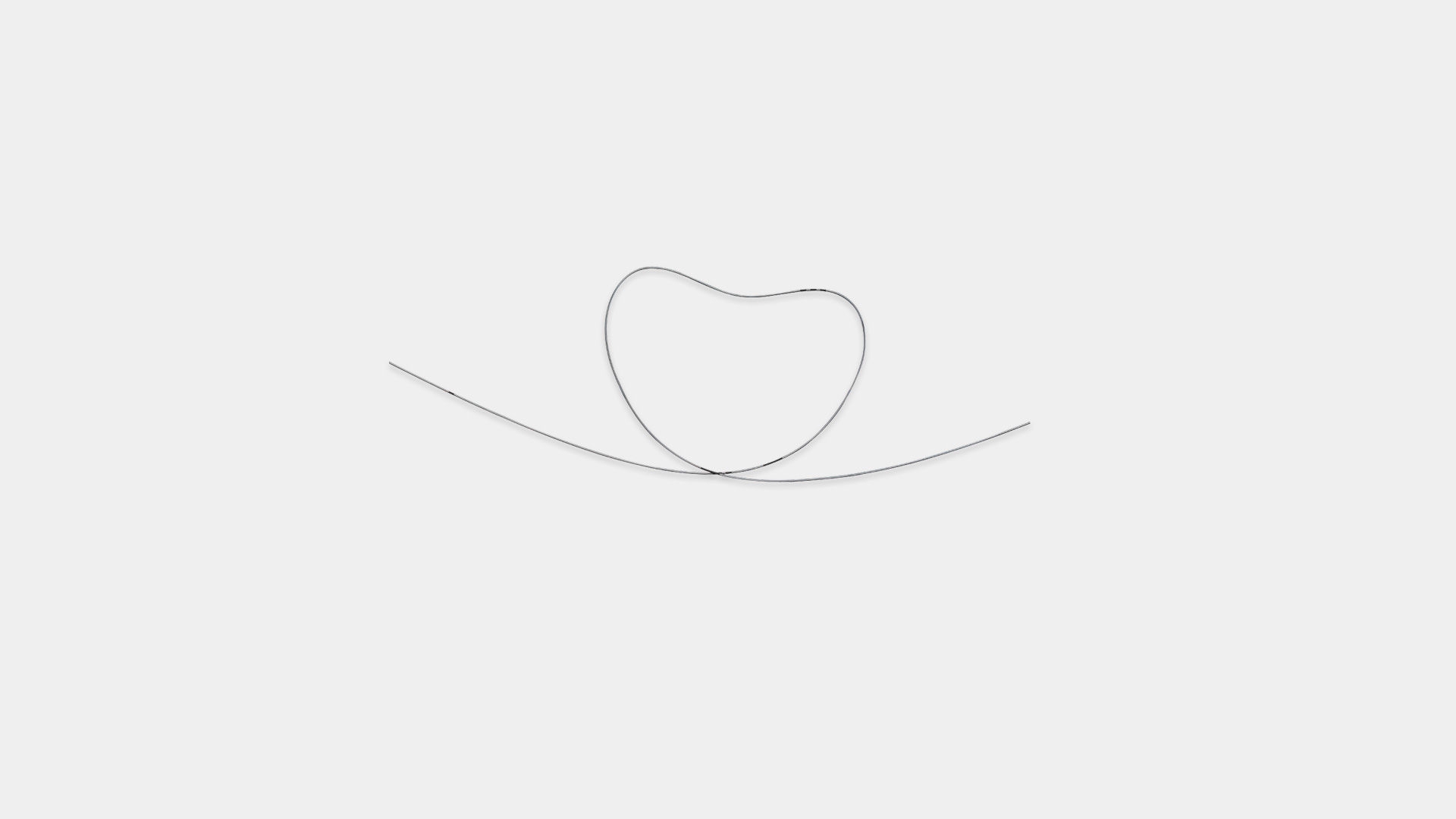
"J" and a straight tip in one wire. Both tips are flexible and suitablefor insertion. All guidewire have length markings.
Flexible ans resilient to knotting to facilitate sucessful insertion og guidewire and placement of CVC.

Certodyn® universal adapter and certodyn® universal adapter paed
Your feedback matters! Participate in our customer survey to help us enhance our website, products and services. Thank you for your support!