No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
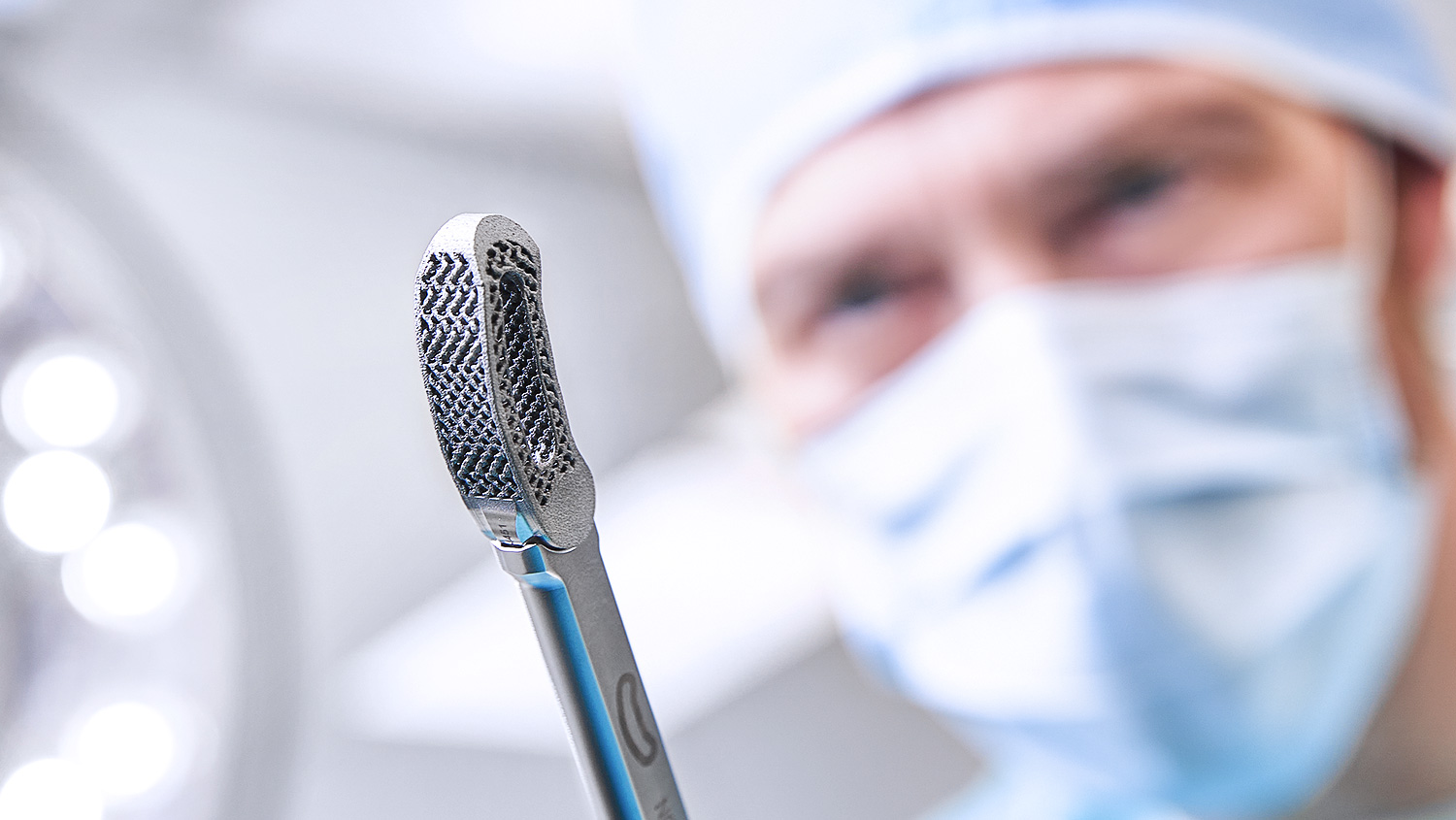
Unfold new spaces for fusion
Inspired by human anatomy, empowered by science – our cages fuse technological advancements with clinical values. The result is a major leap in anterior and posterior stabilization.
Structan®
You think this is an ordinary cage lattice? Well, get impressed by the science behind Structan®. Decades of experience, combined with modern technology, have led to the creation of it – a structure designed for improved clinical outcomes and advanced biomechanical performance.
Surface area is magnified by
0
times, providing more opportunities for bone ingrowth.
Strong and elastic at the same time – Structan® is
0%
closer to the elastic modulus of cortical bone. (1-4) *
Cover posterior stabilization with just
0
modular spinal platform that adapts precisely to your needs.
The finely balanced surface roughness has a positive influence on the adhesion of osteoblasts. The porosity aligns with the human anatomy. This creates a solid basis for bony on-growth and thus fusion with Structan®.[5-8]
/
Substantial osteoblastic differentiation and improved osseointegration – based on scientific evidence, our AESCULAP® 3D Cages reflect the biological attributes of the trabecular structure, empowering bony in-growth.[9-15]
/
Smart designed graft window to support osseointegration between bone and implant – with or without autograft or allograft.
/
The harmonized interface provides a firm connection to instruments and high precision during handling. Feel all of this with our articulating inserter in TLIF procedures – because confidence delivers peace of mind.
/
AESCULAP® 3D interbody fusion devices
As with the creation of all of our spine solutions, the design of the AESCULAP® 3D interbody fusion devices is based on our core values of creating advanced biomechanical performance, intra-operative flexibility and improved clinical outcomes.
Additive manufacturing
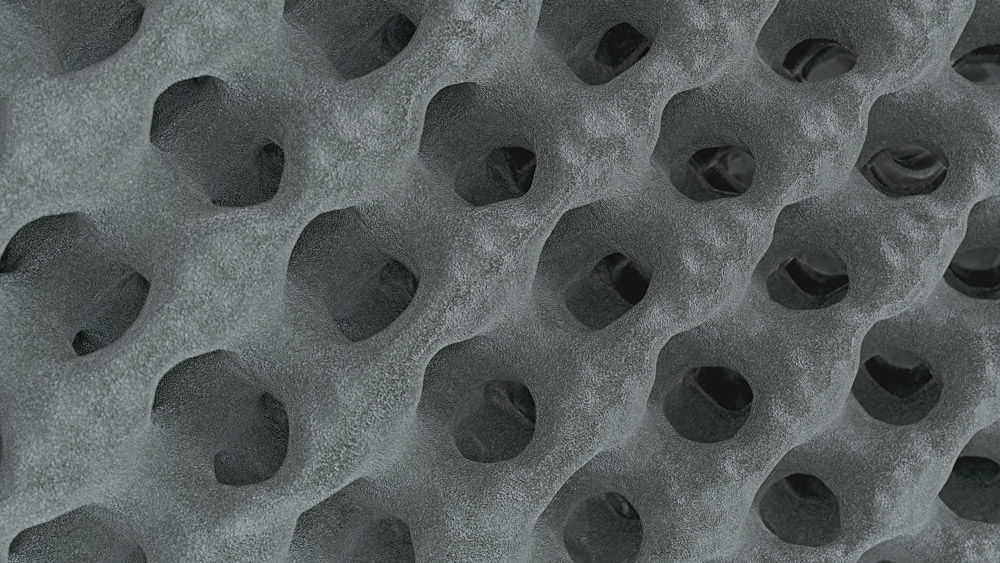
Structan®
The portfolio
Surgical workflow animations
Take a look at the performance of AESCULAP® 3D interbody fusion devices and Ennovate®.
Our TLIF interbody fusion device, with its articulating inserter, enables for true minimally invasive fusion procedures.
With its streamlined surgical technique, our PLIF interbody fusion device and Ennovate® are ideal for the open approach.
Merging the essence of two worlds – our TLIF interbody fusion device can be implanted in a minimally invasive as well open approach.
Discover the AESCULAP® spinal platform
*compared to solid titanium alloy interbody fusion devices.
Your feedback matters! Participate in our customer survey to help us enhance our website, products and services. Thank you for your support!