No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
Pressure ulcer treatment
In most cases, pressure ulcers have a significant impact on patient morbidity[1] and quality of life[2] and can even impact patient survival.[3]
Once a pressure ulcer has developed, it is important to draw up a coordinated treatment plan to promote healing.[4] The basic prerequisites for wound healing must be met and everything that interrupts this process needs to be avoided. These include pressure ease, a clean wound, circulation functioning, an adapted nutrition in terms of both calories and nutrients along with adequate hydration.[5]
 *examples of barriers = slough, necrotic tissue and/or biofilm
*examples of barriers = slough, necrotic tissue and/or biofilm
B. Braun offers different solutions including products for skin care, wound bed preparation and infection management, as well as exudate management. Depending on the extent of tissue damage, pressure sores can be categorized into four stages. Every stage requires a specific treatment.


Linovera® is a product range made of hyperoxygenated fatty acids (HOFA). Indicated for prevention and treatment of stage 1 pressure ulcers (Linovera®) as well as lower limb ulcers and diabetic foot ulcers (Linovera® Emulsion).
The products promote blood microcirculation, reducing the risk of ischemia and facilitating the renewal of epidermal cells. They protect against friction, reduce skin fragility and help to prevent dehydration of the skin.

Askina® Heel is a non adhesive hydrocellular heel dressing that intends to protect the heel area from shear stresses and to reduce pressure from external forces.

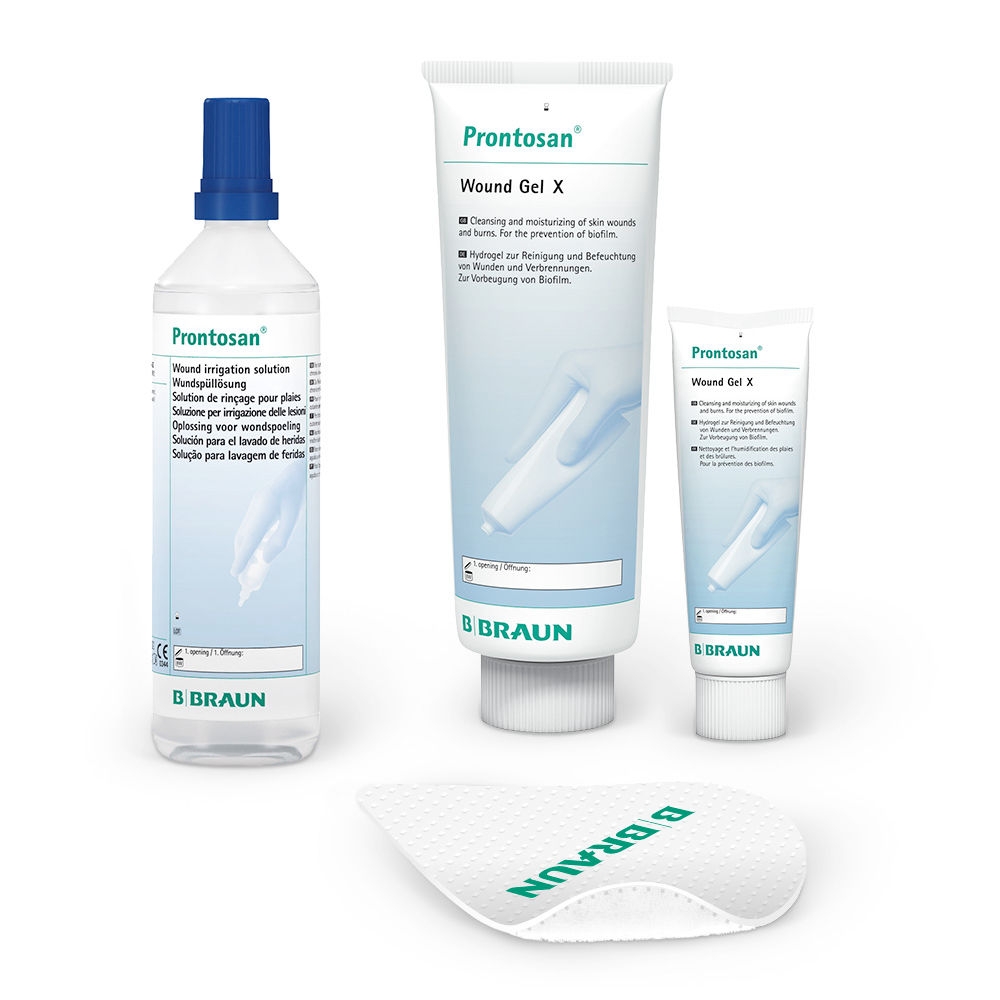
Prontosan® Wound Irrigation Solution is indicated for cleansing, moistening and decontamination of acute and chronic wounds.
The use of Prontosan® Wound Gel X can provide long-lasting cleansing and decontamination of the wound bed between dressing changes.
Prontosan® Debridement Pad has been designed to support the wound bed preparation when used in conjunction with Prontosan® Wound Irrigation Solution.

Askina® Calgitrol® offers a broad antimicrobial effectiveness.

Askina® Carbosorb is a superabsorbent wound dressing with activated charcoal which can manage excess exudate and malodor at the same time.
Askina® DresSil Sacrum, Askina® DresSil Heel, Askina® DresSil, Askina® DresSil Border can help to maintain a moist wound environment conducive to natural healing conditions with a perfored silicone wound contact layer, an absorbent polyurethane foam and a vapor permeable waterproof outer film. Indicated for pressure ulcers (PU), diabetic foot ulcers (DFU), venous leg ulcers (VLU) and 1st/2nd degree burns.
Askina® Foam and Askina® Heel can help to maintain a moist wound environment conducive to natural healing conditions with a polyurethane foam wound contact surface with absorption capacity and a vapor permeable, water and bacteria resistant polyurethane film outer layer.

Askina® Barrier Cream acts as a protectant and moisture barrier against maceration caused by incontinence or bodily fluids, to protect sensitive, fragile skin and severely dry skin, including peri-wound areas. It is indicated for use on intact skin.
Askina® Barrier Film Swab and Askina® Barrier Film Spray are sterile liquid dressings intended to form a uniform, transparent film when applied to the skin. The film can provide oxygen and moisture permeability. Both dressings are indicated for use on intact or damaged skin.

Treatment goals

Prontosan® Wound Irrigation Solution is indicated for cleansing, moistening and decontamination of acute and chronic wounds.
The use of Prontosan® Wound Gel X can provide long-lasting cleansing and decontamination of the wound bed between dressing changes.
Prontosan® Debridement Pad has been designed to support the wound bed preparation when used in conjunction with Prontosan® Wound Irrigation Solution.

Askina® Calgitrol® offers a broad antimicrobial effectiveness.

Askina® Carbosorb is a superabsorbent wound dressing with activated charcoal which can manage excess exudate and malodor at the same time.
Askina® DresSil Sacrum, Askina® DresSil Heel, Askina® DresSil, Askina® DresSil Border can help to maintain a moist wound environment conducive to natural healing conditions with a perfored silicone wound contact layer, an absorbent polyurethane foam and a vapor permeable waterproof outer film. Indicated for pressure ulcers (PU), diabetic foot ulcers (DFU), venous leg ulcers (VLU) and 1st/2nd degree burns.
Askina® Foam and Askina® Heel can help to maintain a moist wound environment conducive to natural healing conditions with a polyurethane foam wound contact surface with absorption capacity and a vapor permeable, water and bacteria resistant polyurethane film outer layer.

Askina® Sorb Rope is an absorbing alginate dressing suited for the management of moderate to heavily exuding cavity wounds.

Askina® Barrier Cream acts as a protectant and moisture barrier against maceration caused by incontinence or bodily fluids, to protect sensitive, fragile skin and severely dry skin, including peri-wound areas. It is indicated for use on intact skin.
Askina® Barrier Film Swab and Askina® Barrier Film Spray are sterile liquid dressings intended to form a uniform, transparent film when applied to the skin. The film can provide oxygen and moisture permeability. Both dressings are indicated for use on intact or damaged skin.


Prontosan® Wound Irrigation Solution is indicated for cleansing, moistening and decontamination of acute and chronic wounds.
The use of Prontosan® Wound Gel X can provide long-lasting cleansing and decontamination of the wound bed between dressing changes.
Prontosan® Debridement Pad has been designed to support the wound bed preparation when used in conjunction with Prontosan® Wound Irrigation Solution.

Askina® Calgitrol® offers a broad antimicrobial effectiveness.

Askina® Carbosorb is a superabsorbent wound dressing with activated charcoal which can manage excess exudate and malodor at the same time.
Askina® DresSil Sacrum, Askina® DresSil Heel, Askina® DresSil, Askina® DresSil Border can help to maintain a moist wound environment conducive to natural healing conditions with a perfored silicone wound contact layer, an absorbent polyurethane foam and a vapor permeable waterproof outer film. Indicated for pressure ulcers (PU), diabetic foot ulcers (DFU), venous leg ulcers (VLU) and 1st/2nd degree burns.
Askina® Foam and Askina® Heel can help to maintain a moist wound environment conducive to natural healing conditions with a polyurethane foam wound contact surface with absorption capacity and a vapor permeable, water and bacteria resistant polyurethane film outer layer.

Askina® Sorb Rope is an absorbing alginate dressing suited for the management of moderate to heavily exuding cavity wounds.

Askina® Barrier Cream acts as a protectant and moisture barrier against maceration caused by incontinence or bodily fluids, to protect sensitive, fragile skin and severely dry skin, including peri-wound areas. It is indicated for use on intact skin.
Askina® Barrier Film Swab and Askina® Barrier Film Spray are sterile liquid dressings intended to form a uniform, transparent film when applied to the skin. The film can provide oxygen and moisture permeability. Both dressings are indicated for use on intact or damaged skin.
Thorough wound assessments and sensible product selections are essential to help prevent and treat wound infections, manage exudate and finally restore skin integrity[4]. This is the clinical case presentation of Fernanda Cortes from Chile who explains step by step how she treated the stage 3 pressure ulcer of a 60-year-old family father. Closely escorted by her commitment and the combination of Prontosan®, Askina® and Linovera® the patient eventually recovered.

For more product information check also current Instructions for use (IFU).
Your feedback matters! Participate in our customer survey to help us enhance our website, products and services. Thank you for your support!