No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
We are proud of it
To serve you with the best treatment for your patients in orthopaedic surgery we take all the challenges and turn them into solutions. It is important for us to deliver implants that allow for the best possible reconstruction of the knee and hip joint.

more than
0
publications since 2000
(released by our internal departments or external cooperation studies)
around
0
tests per year
(of these 40% according to product standards and 60% in custom test set-ups)
currently
0
test rigs
(13 dynamic testing machines, 6 wear simulation testing machines, 1 knee/hip kinematic simulator, 1 static testing machine, 1 universal testing machine (smart tester) for instruments)
We prove the quality promise to our customers with tests, simulations and continuous development, today more than 3,000 test series have been performed – that results meanwhile in more than 150 publications. In our biomechanic laboratory, 14 engineers, scientists and experts set the high quality standard of Aesculap day by day.
To have a realistic simulation of an in-vivo delamination situation, we developed a new test method at the biomechanical laboratory of Aesculap. The method is called highly demanding activities wear simulation, HDA. The test is based on in vivo data collected from patients with total knee endoprosthesis. With HDA we reproduce the activities of an todays average patient in clinical component usage between 15 and 30 years.

“The AESCULAP® knee implant systems had been mechanically tested under many different clinical scenarios. These have included wear testing under clinically relevant high demand activities. The tests ensure that the prostheses withstand loads beyond the standard tests.”
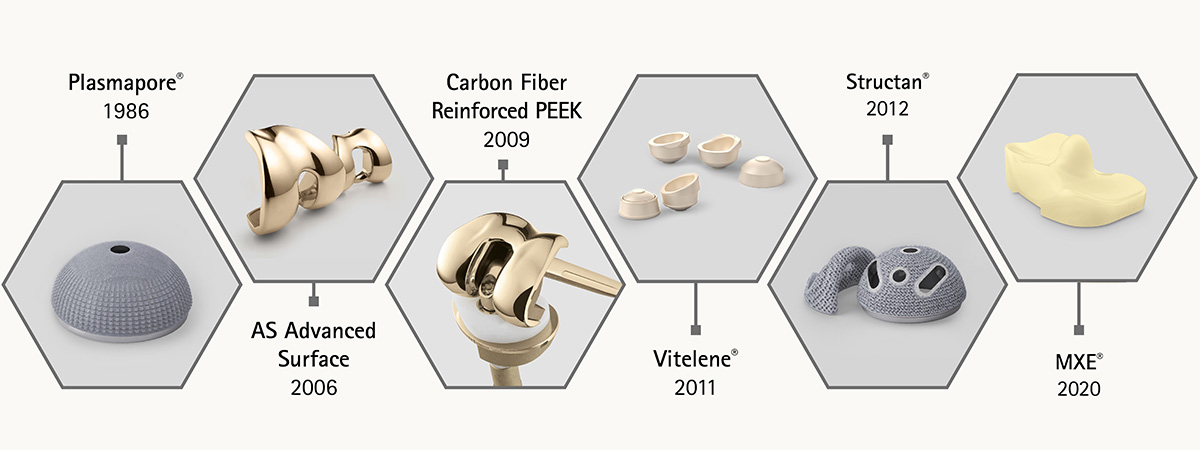
The cementless Plasmapore® implants are coated with a layer of fine titanium. The pore sizes range from 50 to 200 μm with a microporosity of 35% and a thickness of 0.35 mm. These characteristics are optimal for bone ingrowth.
/
This patented technology is available for all of our knee implant systems. The outstanding AS layer architecture makes the prosthesis hard, scratch resistant, reduces wear and the ion release below the detection level.
/
The axis bearings of our EnduRo hinge knee system are made of carbon fibre reinforced PEEK. It provides superior mechanical properties in comparison to PE and we were able to reduce wear by 82% to only 0.21 mm³/mio. cycles.
/
Thanks to its excellent wear and oxidation resistance as well as balanced mechanical properties, Vitelene® represents the latest generation of Vitamin E stabilized highly crosslinked polyethylene for hip replacement.
/
The very rough Structan® profile structure is made of a titanium alloy using additive technology. This additive process allows an all-over regular pore size and porosity in a favorable range to stimulate bone ingrowth.
/
MXE is our latest patented PE material in TKA. Vitamin E protects against delamination and a radiation dose below 50 kGy provides mechanical stability. Doubling the shelf life to 10 years is a great success for the logistics.
/
It will be forwarded to a responsible contact person who will get in touch with you as soon as possible.
Your feedback matters! Participate in our customer survey to help us enhance our website, products and services. Thank you for your support!